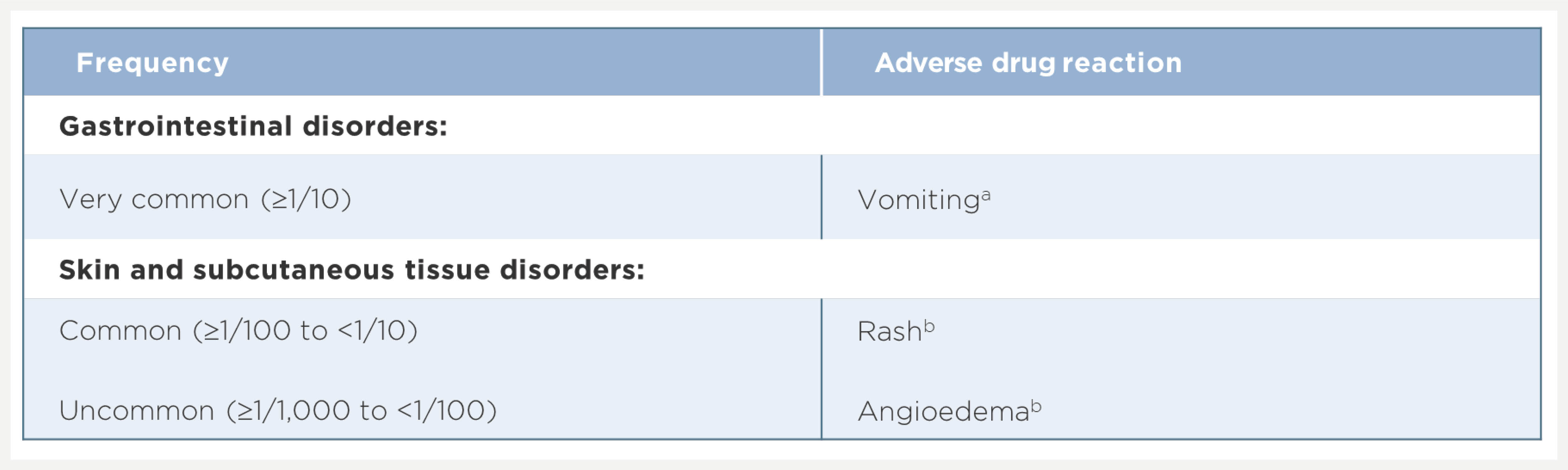
• The proportion of patients who permanently discontinued treatment due to AEs was 0.2%
• The proportion of patients who experienced any severe AE was 3.2%
• Headache, fatigue and nausea were the most common AEs (incidence ≥10%)
• These and other AEs were reported at a similar frequency in patients receiving placebo compared with EPCLUSA® treated patients in the Phase 3 pivotal clinical studies.