EPCLUSA® comes with a one pill, once a day regimen. Co-administration with PPIs is not recommended. If co-administration is necessary, then EPCLUSA® should be administered with food and taken 4 hours before PPI at max doses comparable to omeprazole 20 mg. When EPCLUSA® is administered to adults with decompensated cirrhosis, it is recommended RBV be administered as well. Please refer also to the Summary of Product Characteristics of the medicinal product containing RBV.1
Convenient and consistent dosing for adults HCV patients without compensated cirrhosis1,*,†
One pill, once a day, with no automatic food requirement1,‡
Discreet & convenient storage
EPCLUSA® tablets are supplied in HDPE bottles.1 Suitable for patients with irregular housing, irregular shift patterns and those who are concerned about bulky or noisy blister packaging.
What does simplicity in HCV treatment mean to your patients?
Consistent with data from larger samples in Europe and Canada, UK market research among HCV patients found strong preference for a 12-week single-tablet regimen with no food requirement versus 8-week 3-tablet daily regimen taken with food.2–4
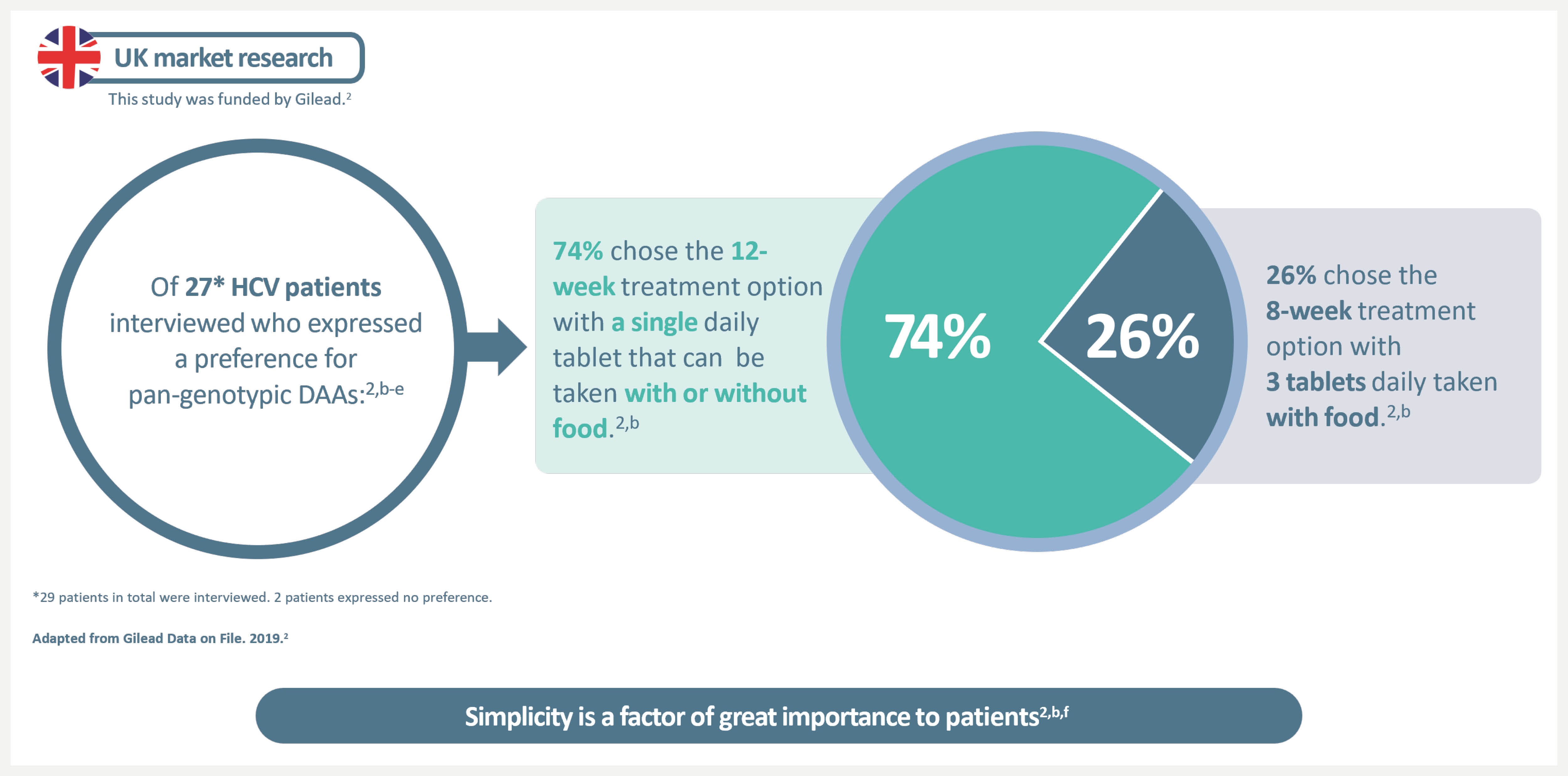
a29 patients in total were interviewed. 2 patients expressed no preference.
bDescriptive analysis of the posology preferences of 29 HCV patients treated with pan-genotypic DAAs (profiles based on EPCLUSA® and glecaprevir/pibrentasvir). This study was funded by Gilead. Product A was a treatment that included a single large tablet daily, which should not be crushed or chewed, and which could be taken at any time of day and without the need for food, for a period of 12 weeks. Product B was a treatment that included 3 large tablets a day in a single dose, which should not be crushed or chewed, and which should be taken at the same time, for a period of 8 weeks.2 The SmPC for glecaprevir/pibrentasvir recommends it be taken with food5
cEPCLUSA® offers an RBV-free STR option for the majority of HCV patients, excluding those with decompensated cirrhosis. For further information on restrictions, please refer to the SmPC. RBV is recommended for the treatment of patients with decompensated cirrhosis and may be considered for the treatment of HCV GT3 patients with compensated cirrhosis1
d24-week EPCLUSA® with RBV may be considered for adult patients who have previously failed therapy with an NS5A-containing regimen1
eNo dose adjustment of EPCLUSA® is required for patients with mild, moderate, or severe hepatic impairment (CPT Class A, B or C). The safety and efficacy of EPCLUSA® have not been assessed in patients with CPT Class C cirrhosis1
fOther factors of importance identified by patients included interactions with substances and flexibility to take tablets with or without food.2
Missing a dose1
It is important that patients take EPCLUSA® every day
If a dose of EPCLUSA® is missed and it is within an 18-hour window of the normal time, patients should be instructed to take the dose as soon as possible and take the next dose at the usual time
If a dose of EPCLUSA® is missed and more than 18 hours has passed then patients should be instructed to wait and take their next dose of EPCLUSA® at the usual time
Patients should be instructed not to take a double dose of EPCLUSA®.
You may also like to visit
Footnotes:
*EPCLUSA® offers an RBV-free STR option for the majority of HCV patients, excluding those with decompensated cirrhosis. For further information on restrictions please refer to the SmPC. Addition of RBV is recommended for the treatment of patients with decompensated cirrhosis and may be considered for the treatment of HCV GT3 patients with compensated cirrhosis.1 The definition of single-tablet regimen or STR on this webpage is: one tablet, once daily
†24-week EPCLUSA® with RBV may be considered for patients who have previously failed therapy with an NS5A-containing regimen1
‡Except when coadministered with PPIs or in combination with RBV. Refer to EPCLUSA® SmPC for further information.1
Abbreviations:
CPT = Child-Pugh-Turcotte; DAA = direct-acting antiviral; HCV = hepatitis C virus; HDPE = High-density polyethylene; GT = genotype; NS5A = nonstructural protein 5A; PPI = proton pump inhibitor; RBV = ribavirin; STR = single tablet regimen.
References:
- EPCLUSA® Summary of Product Characteristics.
- Gilead. Data on file. 2019.
- Robertson et al. J Canadian Market Research. Gilead. 2018.
- Simón M, et al. Patients' preferences for treatment with the new direct acting antiviral therapies for chronic hepatitis C virus infection. Rev Esp Sanid Penit. 2021;23:67–75.
- Glecaprevir/Pibrentasvir Summary of Product Characteristics.
UK-EPC-0468
Date of preparation July 2024

