EPCLUSA® delivers high real-world SVR rates across genotypes1,*
Providing confidence across a wide range of patients
aAn open-label, single-arm Phase 4 trial evaluating 12-week treatment with EPCLUSA® in chronic HCV GT1–6 patients with recent injection drug use (past 6 months) from 19 sites across 7 countries. Adherence was monitored through use of an electronic blister pack, return of which by the participant was incentivised. The most frequent AEs were fatigue, headache and nausea. AE-related treatment discontinuations were reported in 1% (1/103) of patients. Treatment-related AEs were seen in 48 (47%) patients (one grade 3, no grade 4). Seven (7%) patients had at least one serious AE; only one such event (rhabdomyolysis, resolved) was possibly related to the therapy.2
Pan-genotypic
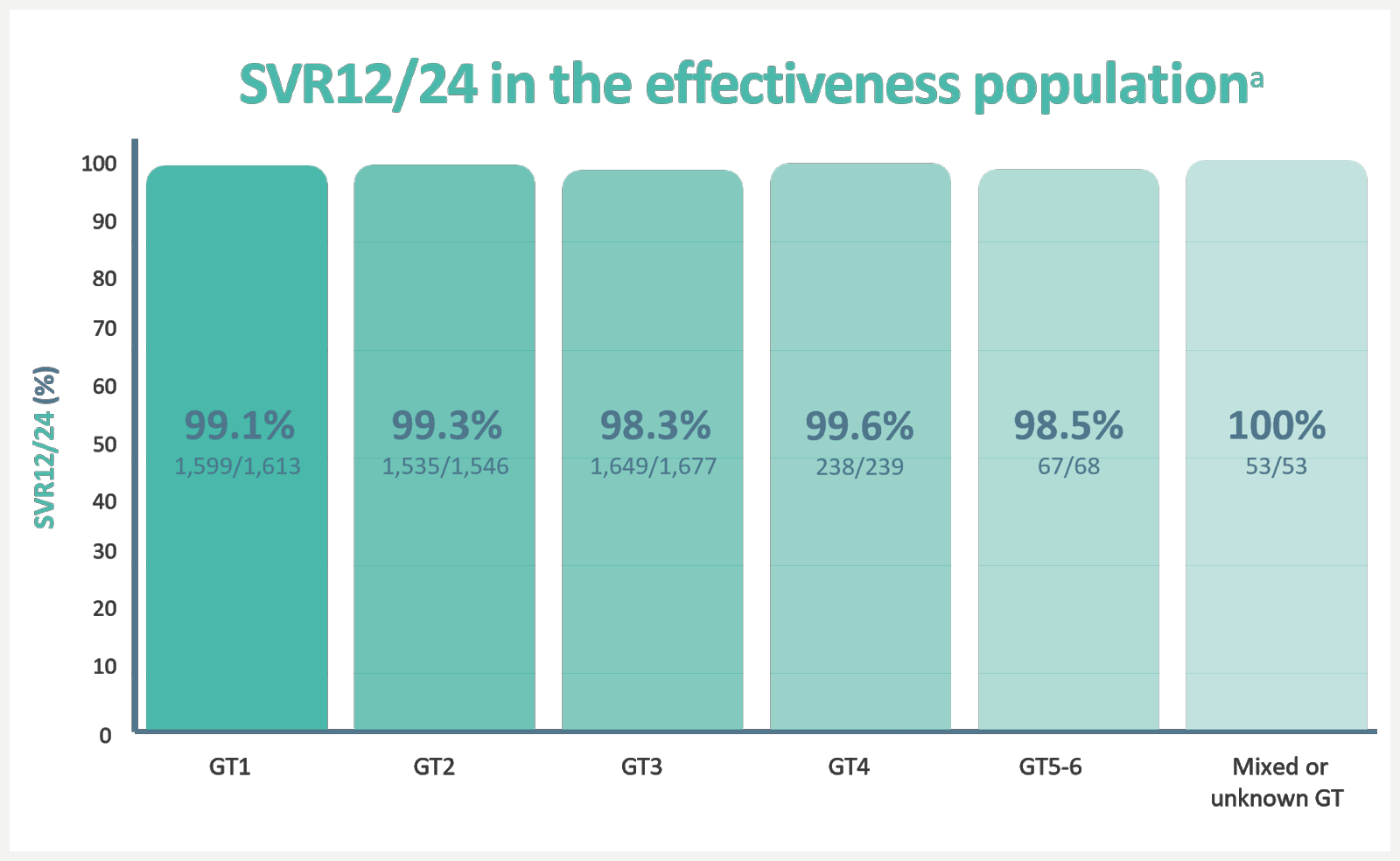
Real-world analysis of 5,552 patients from 12 clinical practice cohorts, across 7 countries.1,b
- Virologic failures: 1% (55/5,552)1
- Non-virologic failures: 6% (332/5,552)1
- Lost to follow-up 67%
- Early discontinuation 27%
- Other reasons 7%
aSVR12/24, sustained virological response at 12 weeks/24 weeks after treatment. The effectiveness population excluded patients who did not achieve SVR12/24 due to non-virological or unknown reasons. SVR12/24 in the overall population was 92.6%1
bA retrospective, pooled analysis of SVR12/24 in adult patients treated for 12 weeks with EPCLUSA® without RBV in 12 real-world cohorts in Canada, Europe and the US (N=5,552). Patients were treated in different clinical settings, including university hospitals, academic centres, community centres, outpatient clinics and private practices. Treatment and patient monitoring were based on local clinical practice and standard of care, at the discretion of the treating physician. Treatment-naïve patients and patients who have previously received IFN-based therapy (Peg-IFN + RBV with or without telaprevir, boceprevir or simeprevir) were included. Patients who had previously been unsucessfully treated with other DAA treatments were excluded from the study. Patients with current or prior decompensated cirrhosis or hepatocellular carcinoma were also excluded from the study.1
Adherence
Adherence is a major factor in achieving cure; and patients can often struggle to stay on treatment while balancing life challenges. With EPCLUSA®, you have a drug you can rely on to deliver high cure rates in the face of uncertainty.2
EPCLUSA® delivered cure rates of 94% for people who inject drugs with a range of adherence with no reported cases of virologic failure2
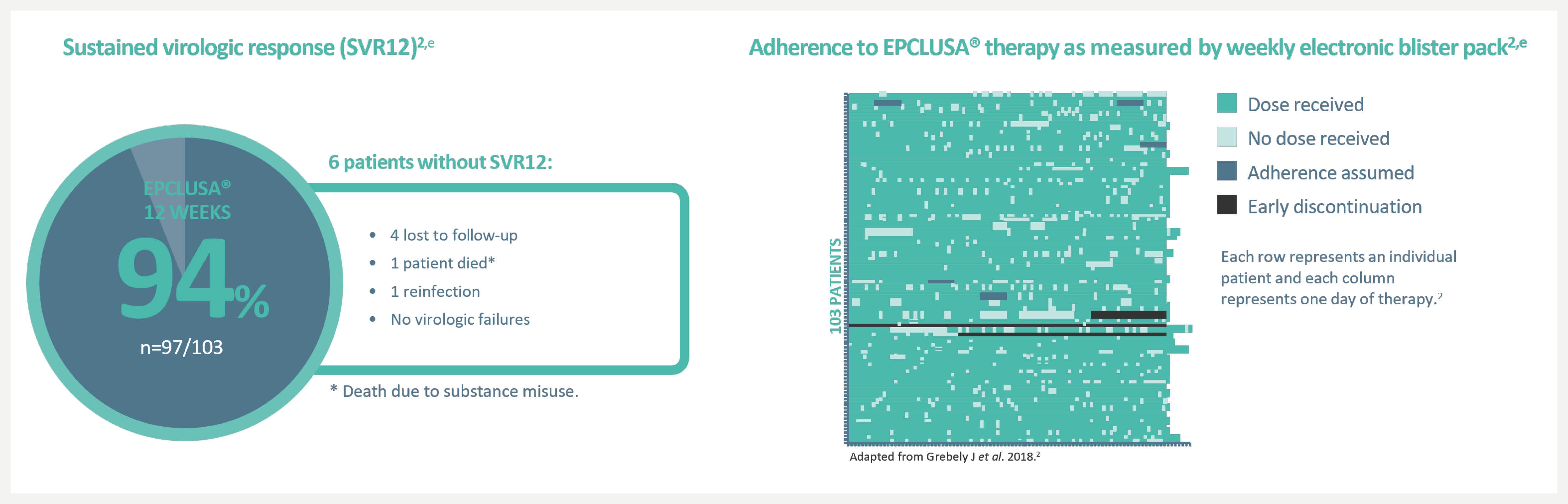
aAn open-label, single-arm Phase 4 trial evaluating 12-week treatment with EPCLUSA® in chronic HCV GT1–6 patients with recent injection drug use (past 6 months) from 19 sites across 7 countries. Adherence was monitored through use of an electronic blister pack, return of which by the participant was incentivised. The most frequent AEs were fatigue, headache and nausea. AE-related treatment discontinuations were reported in 1% (1/103) of patients. Treatment-related AEs were seen in 48 (47%) patients (one grade 3, no grade 4). Seven (7%) patients had at least one serious AE; only one such event (rhabdomyolysis, resolved) was possibly related to the therapy.2
bDosage of EPCLUSA® is one tablet, taken orally once daily with or without food for 12 weeks in patients without cirrhosis and patients with compensated cirrhosis.3
DDIs
DDIs can add unwanted uncertainties when initiating HCV treatment after diagnosis. When a person living with HCV relies on treatment for a chronic condition, you can’t predict how they’ll react when you introduce another medicine. People living with HCV have a higher burden of comorbidities than individuals without HCV.6 Comorbidities can vary according to each person and their lifestyle and can pose a challenge to effective treatment. Some people living with HCV might not be able to provide a full medical history and identify all medications they might be on during their treatment.7 EPCLUSA® has a well-documented DDI profile, with few clinically relevant DDIs.3,4,7,† Please refer to EPCLUSA® SmPC for further information.
No clinical advice is given or implied, clinicians must exercise their own judgement in relation to the risks and benefits of combining drugs, which depend on factors beyond pharmacokinetic interactions between two drugs.
Susbstance use
When substance use is a concern, EPCLUSA® has no known interactions with opioids, including oxycodone, fentanyl and OST.4
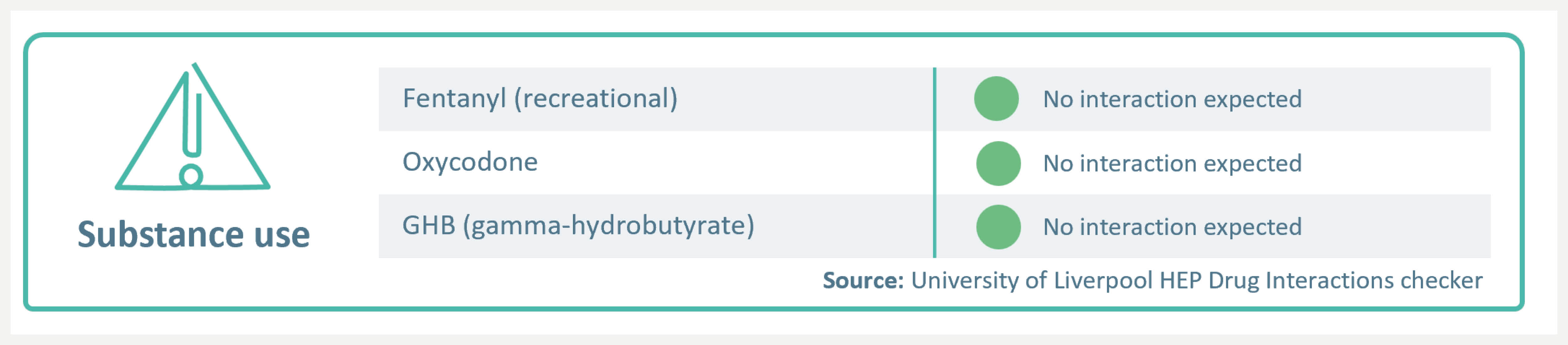
Please refer to the SmPC to check for interactions before prescribing. EPCLUSA® is contraindicated for use with strong P-gp and/or strong CYP inducers, such as St John’s wort.3 For a full list of DDIs please refer to the SmPC and visit: https://www.hep-druginteractions.org/checker.
Mental health
High cure rates with EPCLUSA® were not impacted by the presence of mental health disorders and/or antipsychotic treatment.8 No known interactions with aripiprazole, quetiapine, or sertraline.4
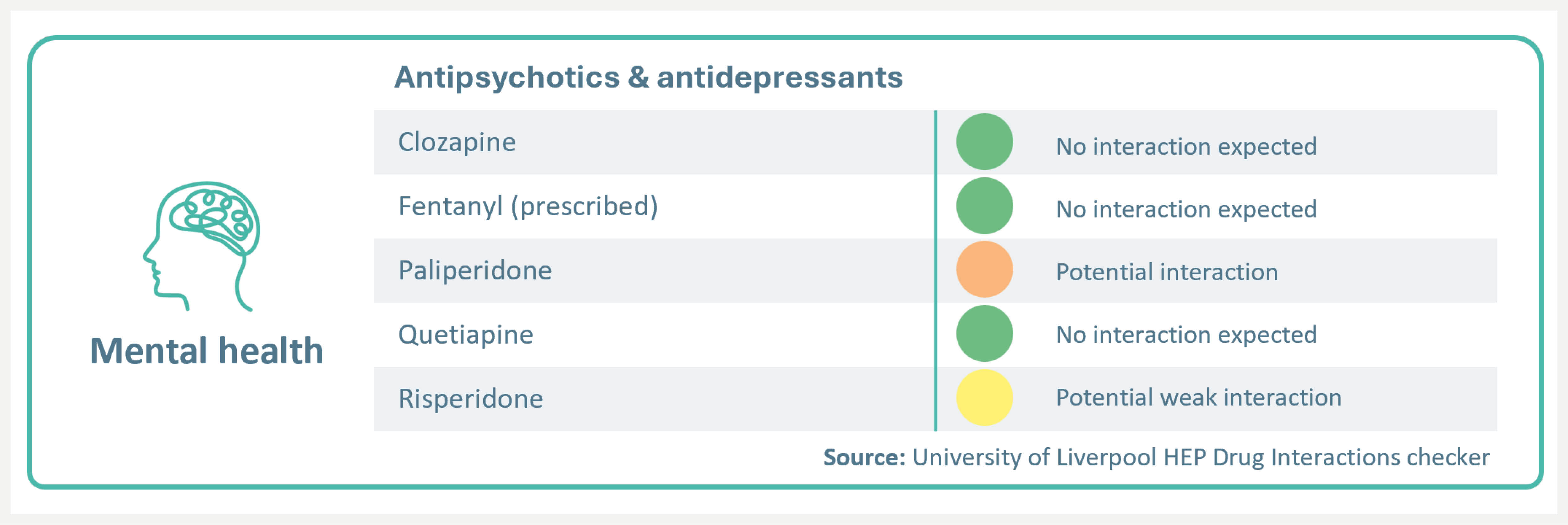
EPCLUSA® has a potential interaction with paliperidone (no a priori dose adjustment required; additional monitoring may be required) and potential weak interaction with risperidone (no dose adjustment or additional monitoring is required)4
For a full list of DDIs please refer to the SmPC and visit: https://www.hep-druginteractions.org/checker.
Statin use
EPCLUSA® has no contraindications with statins.3,4 With appropriate monitoring and dose reduction, patients may continue using statins concomitantly with EPCLUSA®.3,4
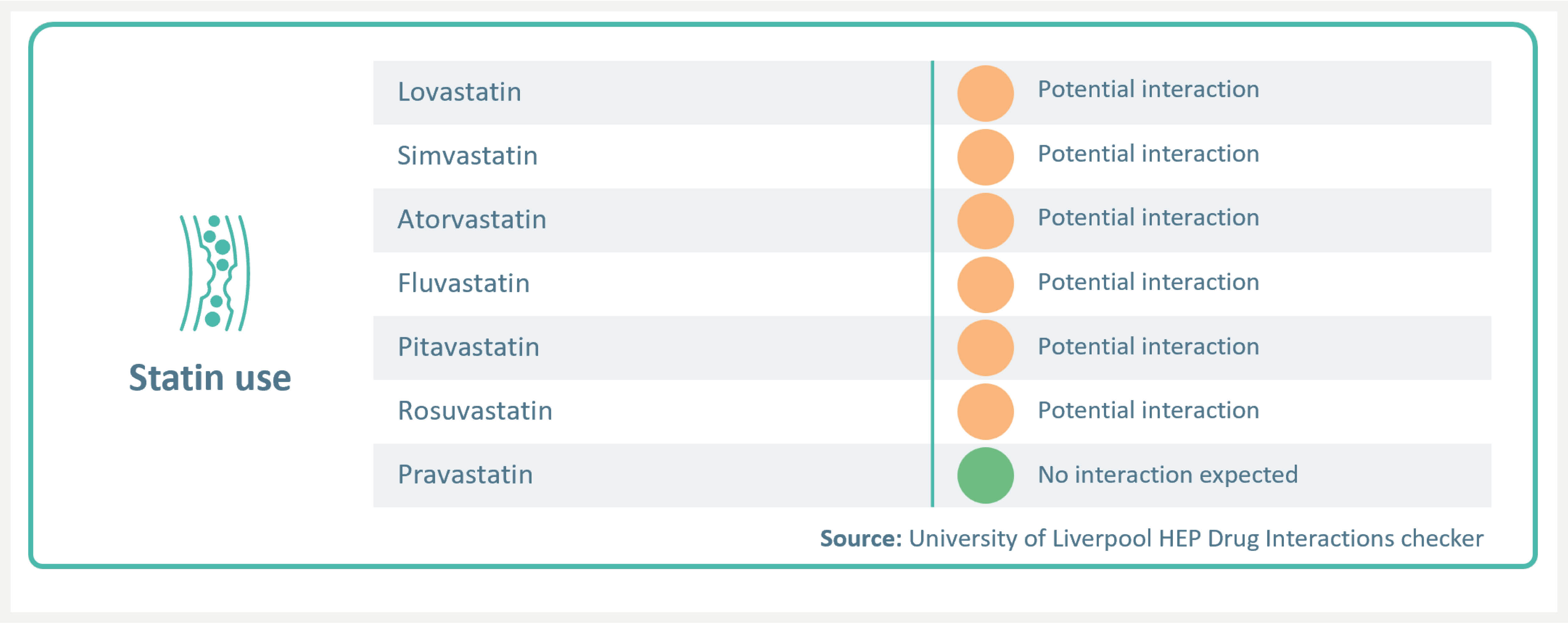
EPCLUSA® has a potential interaction with fluvastatin, lovastatin, pitavastatin, simvastatin, atorvastatin, and rosuvastatin. Statin-associated adverse events such as myopathy should be closely monitored, and dose reduction may be required.4 For a full list of DDIs please refer to the SmPC and visit:
https://www.hep-druginteractions.org/checker.
You may also like to visit
Footnotes:
*EASL defines cure as SVR, i.e. undetectable HCV RNA after treatment completion.
†Analysis of DDI risk in patients prescribed DAAs in 2018. Data were collected from a proprietary US EMR database covering 21 healthcare organisations and 26 million patients.7
Please refer to the SmPC to check for interactions before prescribing.
Abbreviations:
AE = adverse event; DAA = direct-acting antiviral; DDI = drug-drug interaction; EASL = European Association for Study of the Liver; GT = genotype; HCV = hepatitis C virus; IFN = interferon; OST = opioid substitution therapy; RBV = ribavirin; RNA = ribonucleic acid; SVR = sustained virologic response.
References:
- Mangia A, et al. Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts. Liver Int. 2020;40:1841–1852.
- Grebely J, et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:153–161.
- EPCLUSA® Summary of Product Characteristics.
- University of Liverpool. HEP Drug Interactions Checker. Available from: https://www.hep-druginteractions.org/checker. Accessed July 2024.
- European Association for the Study of the Liver (EASL). EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73:1170–1218.
- Hudson, B et al. Comorbidities and medications of patients with chronic hepatitis C under specialist care in the UK. J Med Virol. 2017;89:2158-2164.
- Curry, MP et al. Poster THU-435 presented at International Liver Congress 2020.
- Davidson K, et al. EASL 2020; poster# THU424.
UK-EPC-0468
Date of preparation July 2024

