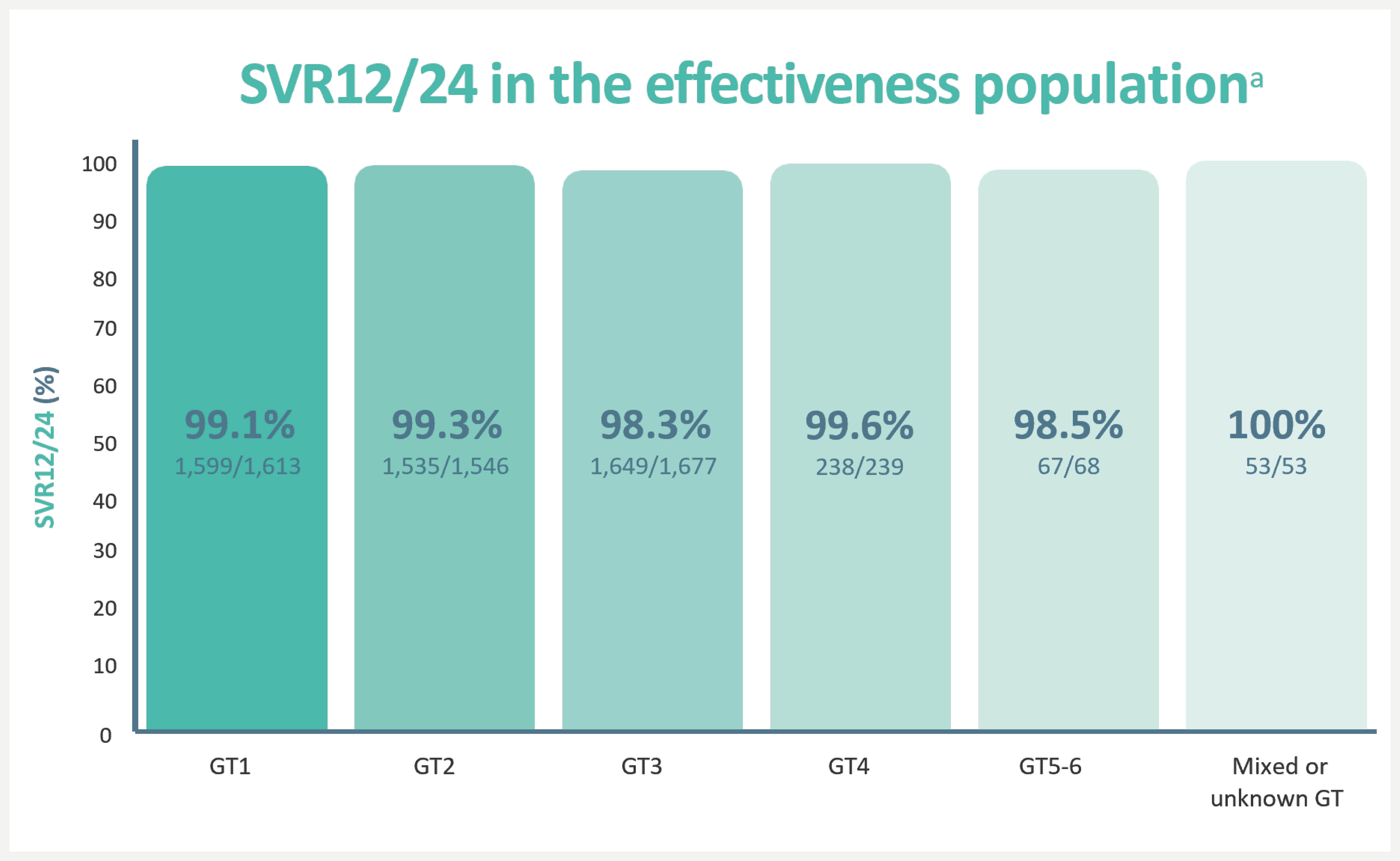
In a real-world clinical analysis from 5,552 patients from 12 clinical practice cohorts across different settings in seven countries in Europe and North America, high SVR rates were observed with EPCLUSA® across genotypes.7,*,†
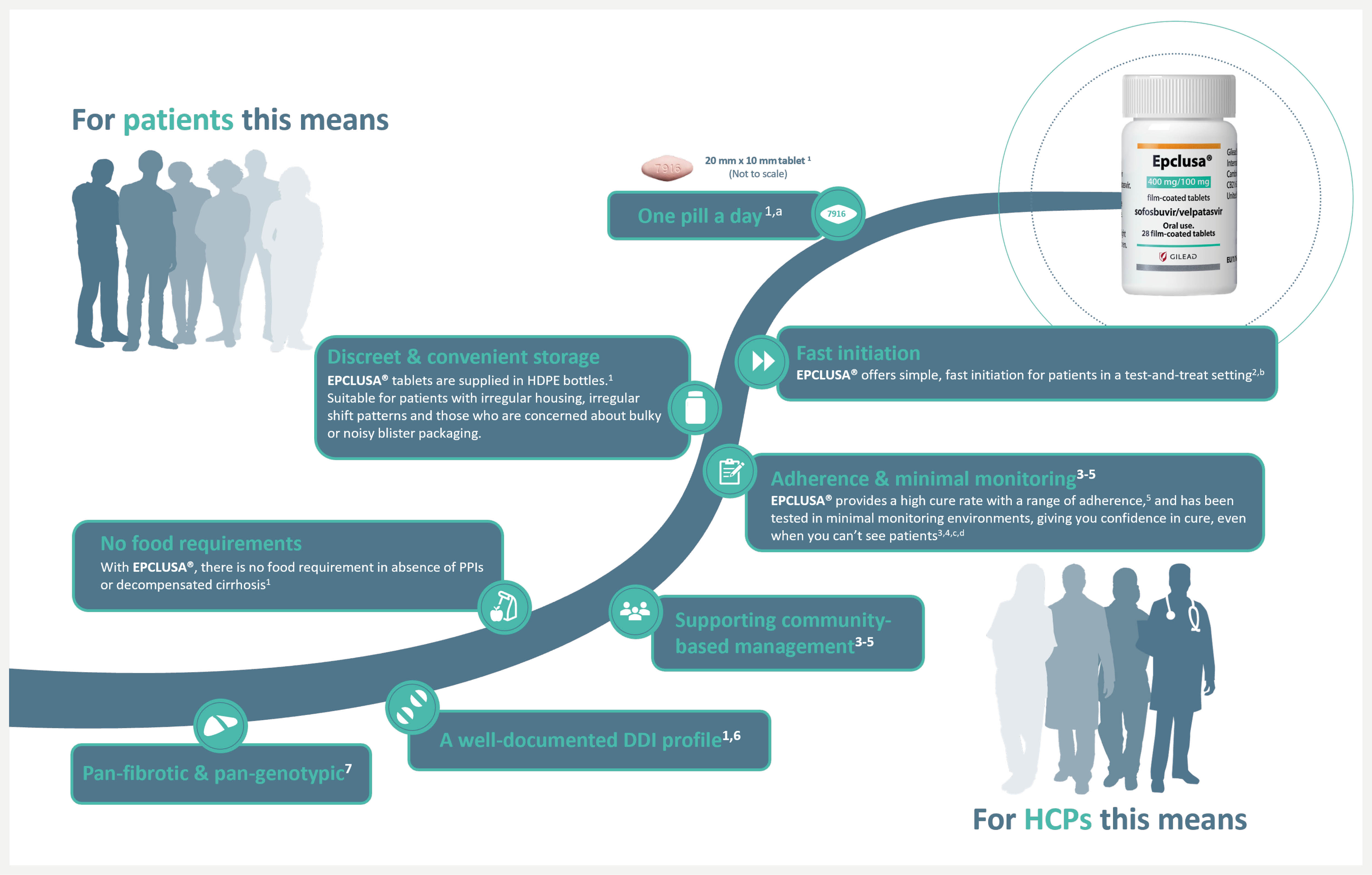
aDosing: EPCLUSA® comes with a one pill, once a day regimen. Co-administration with PPIs is not recommended. If co-administration is necessary, then EPCLUSA® should be administered with food and taken 4 hours before PPI at a max doses comparable to omeprazole 20 mg. When EPCLUSA® is administered to adults with decompensated cirrhosis, it is recommended RBV be administered as well. Please refer also to the Summary of Product Characteristics of the medicinal product containing RBV.1
bFast initiation: In a retrospective integrated analysis from 20 prison settings in Canada and Europe, 526 people in prison infected with HCV genotype 1–6, with or without compensated cirrhosis and who were treated with EPCLUSA® 400/100 mg for 12 weeks without RBV as part of routine clinical practice, were eligible for inclusion.2 In the entire population, SVR was achieved by 98.9% of patients (437/442), including 98.7% (379/384) of treatment-naïve and 100% (51/51) of treatment-experienced (DAA-naïve) patients. SVR rates were numerically similar between patients who received treatment soon after diagnosis and those in whom treatment was delayed: SVR was achieved by 97.7% (42/43) of patients who received treatment within 1 day of diagnosis, 98.4% (60/61) of patients who received treatment within 7 days, 98.7% (236/239) of patients who received treatment within 90 days and 98.6% (145/147) of patients who received treatment more than 90 days after diagnosis.2
cAdherence & minimal monitoring: In a Phase 4 multinational, open-label, prospective, single-arm, interventional study across a broad population (N=399) from 5 countries – USA (n=131), Brazil (n=131), South Africa (n=12), Uganda (n=15) and Thailand (n=110) – EPCLUSA® was tested in minimal monitoring environments for HCV treatment and demonstrated up to 95% cure rate (379/399) (SVR). The patient population included patients with compensated cirrhosis (9%), current/former history of substance use (57%), women (35%) and patients with HIV (42%). There was no pre-treatment genotyping and liver disease stage was determined by FIB-4 assessment. All 84 tablets for 12 weeks of treatment with EPCLUSA® were dispensed at initiation. Monitoring was done remotely at Weeks 4 and 22 (SVR scheduling) with no on-treatment clinic visits.3,4
dAn open-label, single-arm Phase 4 trial evaluating 12-week treatment with EPCLUSA® in chronic HCV GT1–6 patients with recent injection drug use (past 6 months) from 19 sites across 7 countries. Adherence was monitored through use of an electronic blister pack, return of which by the participant was incentivised. The most frequent AEs were fatigue, headache and nausea. AE-related treatment discontinuations were reported in 1% (1/103) of patients. Treatment-related AEs were seen in 48 (47%) patients (one grade 3, no grade 4). Seven (7%) patients had at least one serious AE; only one such event (rhabdomyolysis, resolved) was possibly related to the therapy.5
In a real-world clinical analysis from 5,552 patients from 12 clinical practice cohorts across different settings in seven countries in Europe and North America, high SVR rates were observed with EPCLUSA® across genotypes.7,*,†
Virologic failures: 1% (55/5,552)7
Non-virologic failures: 6% (332/5,552)7
- Lost to follow-up: 67%
- Early discontinuation: 27%
- Other reasons: 7%
aSVR12/24, sustained virological response at 12 weeks/24 weeks after treatment. The effectiveness population excluded patients who did not achieve SVR12/24 due to non-virological or unknown reasons. SVR12/24 in the overall population was 92.6%.7
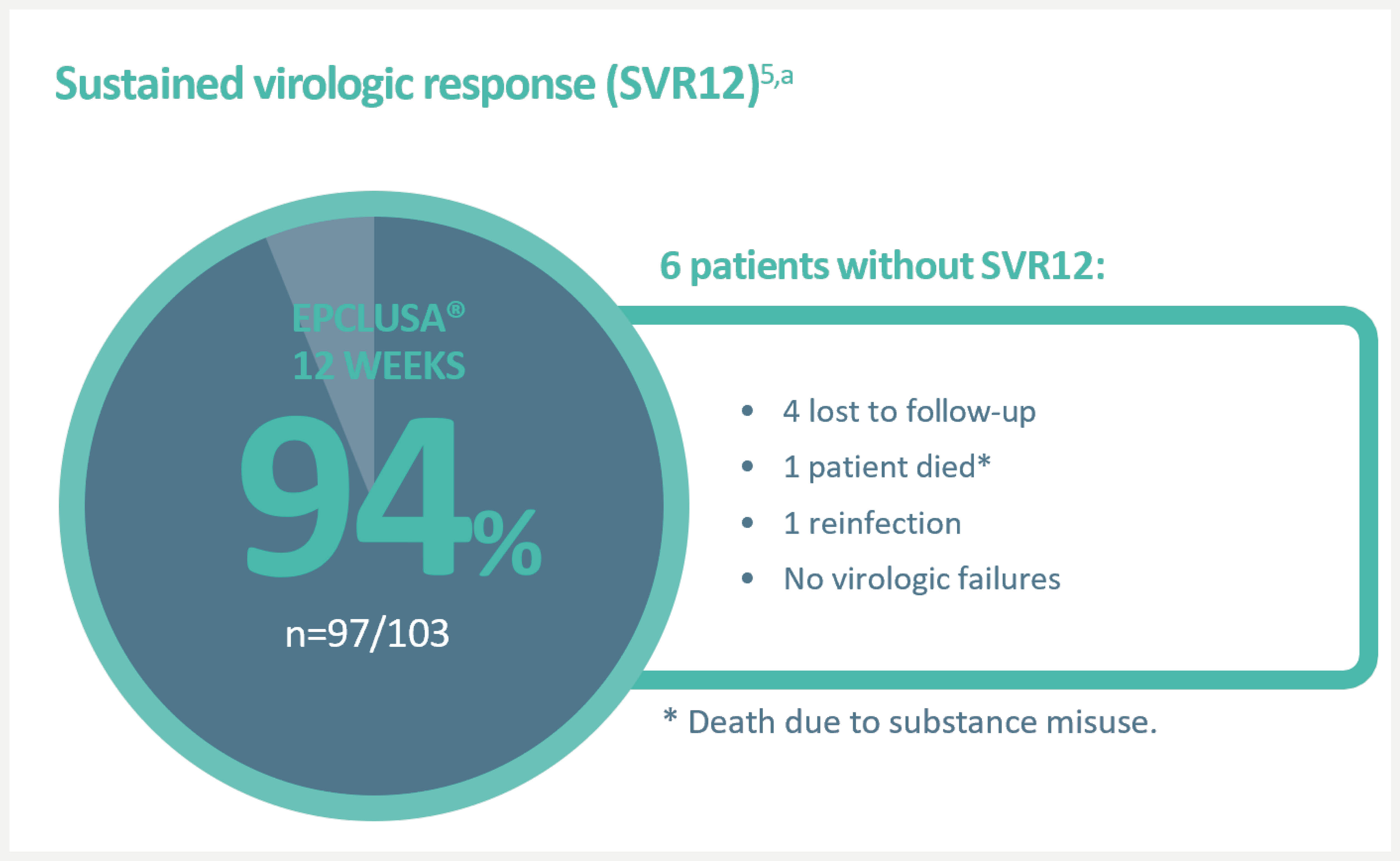
An open-label, single-arm Phase 4 trial evaluating 12-week treatment with EPCLUSA® in chronic HCV GT1–6 patients with recent injection drug use (past 6 months) from 19 sites across 7 countries.5,‡
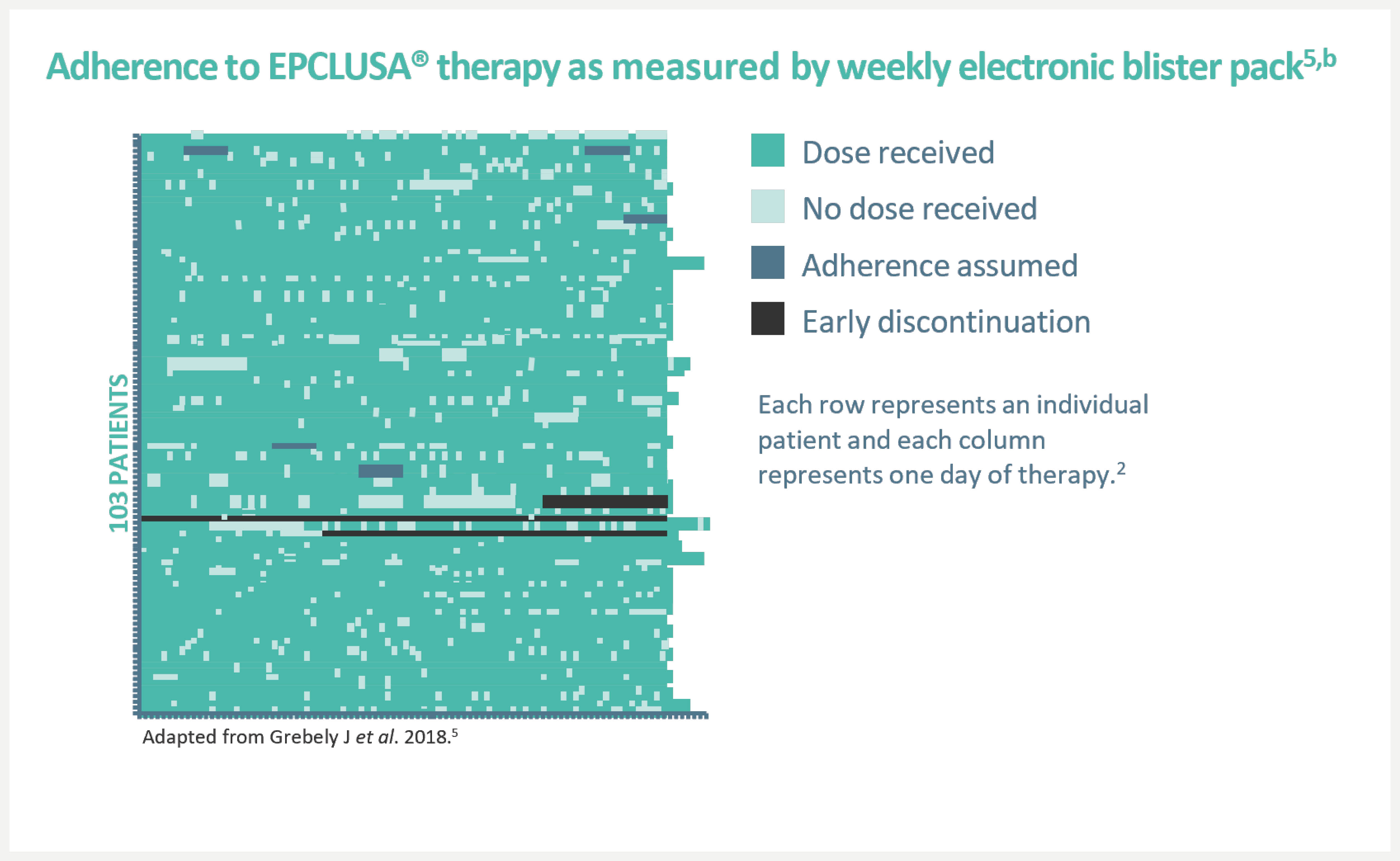
People with HCV may experience issues with substance misuse and housing, making returning for follow-up appointments more challenging for them. With EPCLUSA®, you can deliver high cure rates in community settings that may be more accessible to these patients.3,4
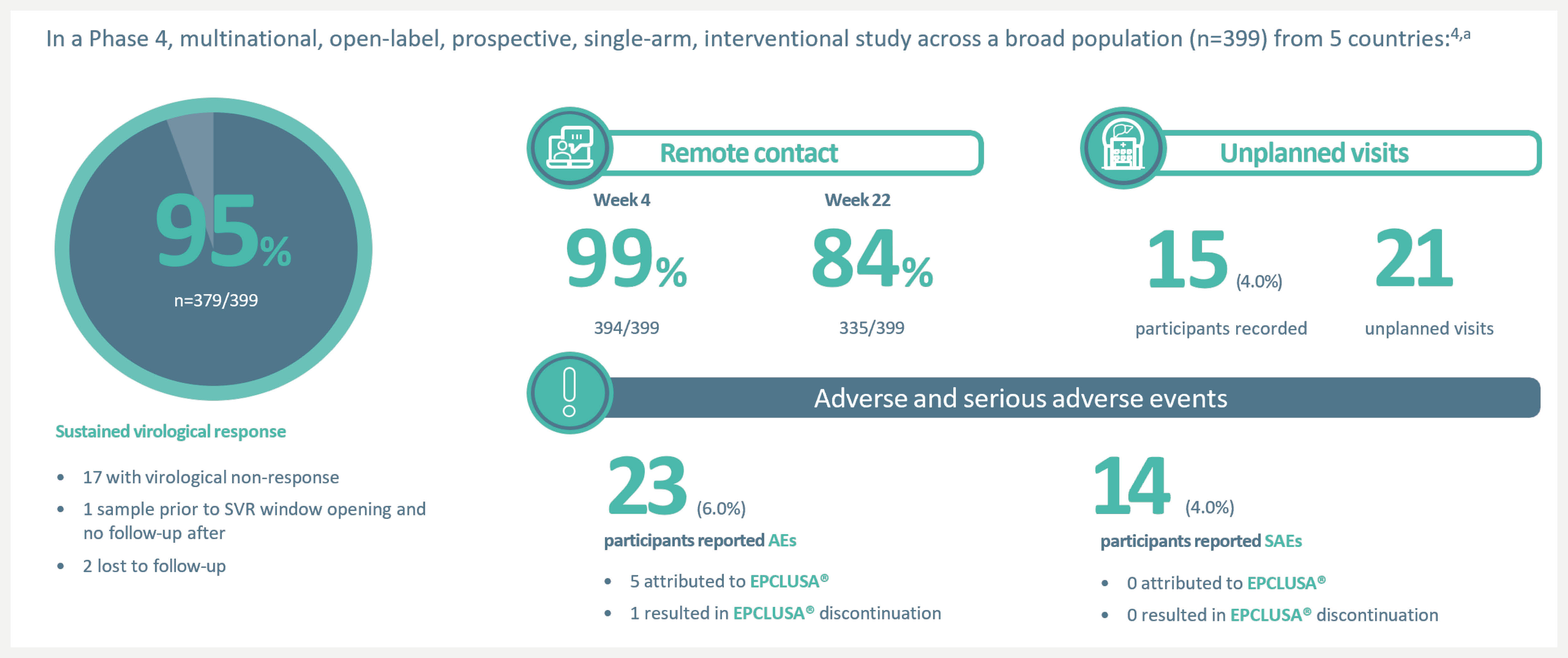
aIn a Phase 4 multinational, open-label, prospective, single-arm, interventional study across a broad population (n=399) from 5 countries – USA (n=131), Brazil (n=131), South Africa (n=12), Uganda (n=15) and Thailand (n=110) – EPCLUSA® was tested in minimal monitoring environments for HCV treatment and demonstrated up to 95% cure rate (379/399) (SVR). The patient population included patients with compensated cirrhosis (9%), current/former history of substance use (57%), women (35%) and patients with HIV coinfection (42%). There was no pre-treatment genotyping and liver disease stage was determined by FIB-4 assessment. All 84 tablets for 12 weeks of treatment with EPCLUSA® were dispensed at initiation. Monitoring was done remotely at Weeks 4 and 22 (SVR scheduling) with no on-treatment clinic visits.4
bA UK patient population was not included in this study and therefore the baseline demographics of the patients studied (e.g. 42% HIV coinfection, 57% former/current history of substance use) are not closely representative of the UK population.4
cFIB-4 liver assessment and no pre-treatment genotyping. All 84 tablets for 12 weeks of treatment with EPCLUSA® were dispensed at initiation. Monitoring was done remotely at Week 4 and 22 (SVR scheduling) with no on-treatment clinic visits.4 Patients also taking apixaban, atorvastatin, carvedilol, dabigatran, digoxin, diltiazem, edoxaban, fluvastatin, lovastatin, pitavastatin, rivaroxaban, rosuvastatin, simvastatin, tenofovir disoproxil fumarate (TDF), ticagrelor, or warfarin may require adjustments to dose size or timing.6
*EASL defines cure as SVR, i.e. undetectable HCV RNA after treatment completion6
†A retrospective, pooled analysis of SVR12/24 in adult patients treated for 12 weeks with EPCLUSA® without RBV in 12 real-world cohorts in Canada, Europe and the US (N=5,552). Patients were treated in different clinical settings, including university hospitals, academic centres, community centres, outpatient clinics and private practices. Treatment and patient monitoring were based on local clinical practice and standard of care, at the discretion of the treating physician. Treatment-naïve patients and patients who have previously received IFN-based therapy (Peg-IFN + RBV with or without telaprevir, boceprevir or simeprevir) were included. Patients who had previously been unsucessfully treated with other DAA treatments were excluded from the study. Patients with current or prior decompensated cirrhosis or hepatocellular carcinoma were also excluded from the study7
‡An open-label, single-arm Phase 4 trial evaluating 12-week treatment with EPCLUSA® in chronic HCV GT1–6 patients with recent injection drug use (past 6 months) from 19 sites across 7 countries. Adherence was monitored through use of an electronic blister pack, return of which by the participant was incentivised. The most frequent AEs were fatigue, headache and nausea. AE-related treatment discontinuations were reported in 1% (1/103) of patients. Treatment-related AEs were seen in 48 (47%) patients (one grade 3, no grade 4). Seven (7%) patients had at least one serious AE; only one such event (rhabdomyolysis, resolved) was possibly related to the therapy.5
AE = adverse event; DAA = direct-acting antiviral; DDI = drug–drug interaction; EASL = European Association for the Study of the Liver; FIB-4 = Fibrosis-4; GT = genotype; HCP = healthcare provider; HCV = hepatitis C virus; HDPE = High-density polyethylene; PPI = proton-pump inhibitor; RBV = ribavirin; SVR = sustained virologic response.
UK-EPC-0468
Date of preparation July 2024