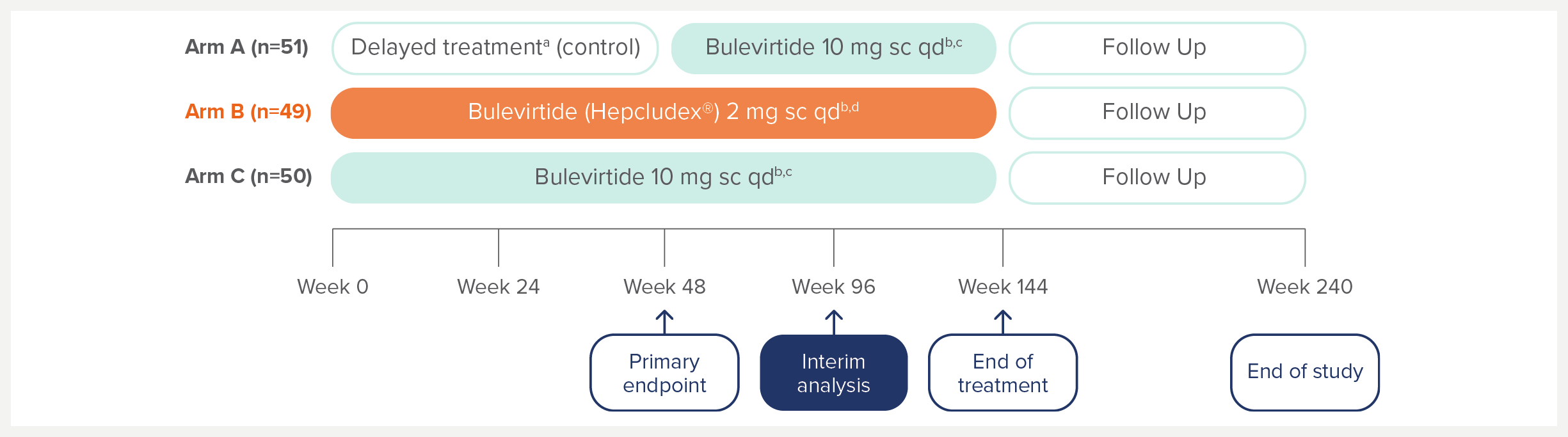
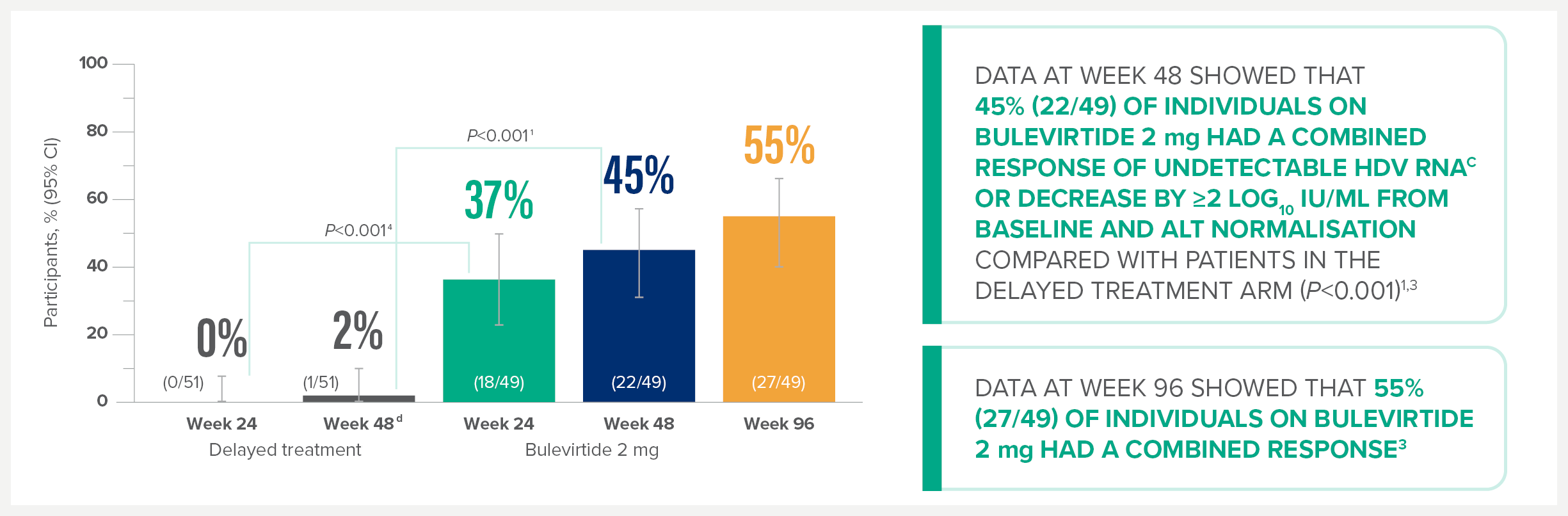
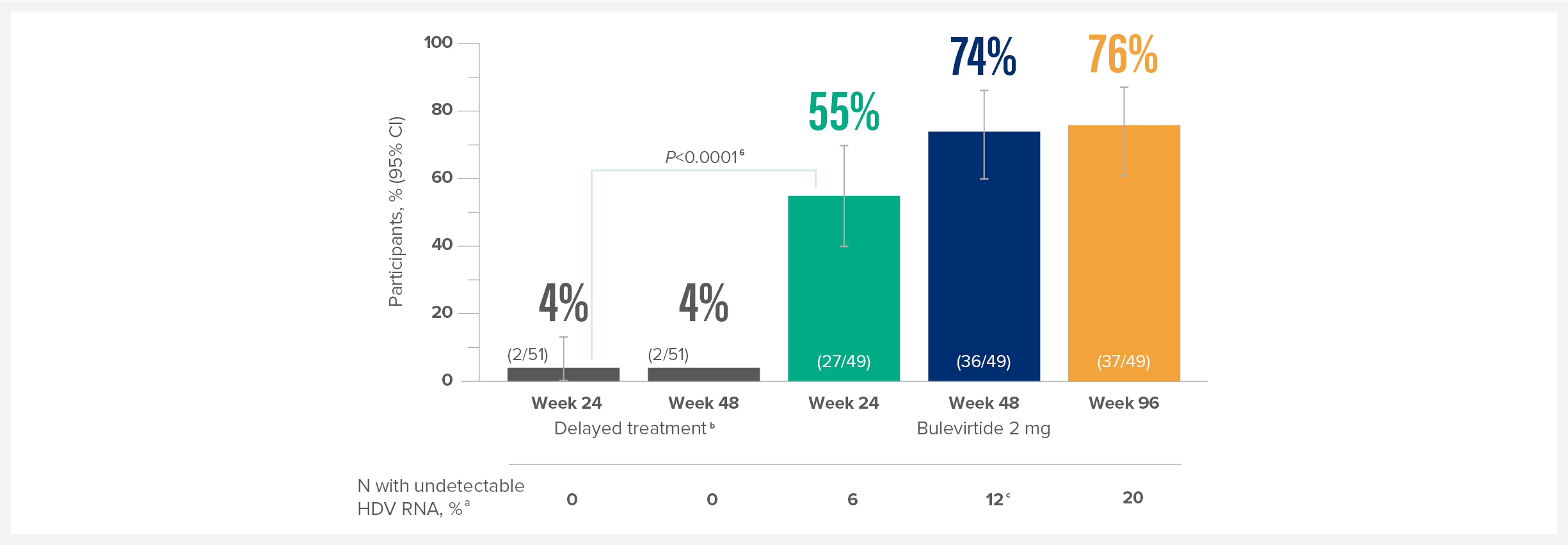
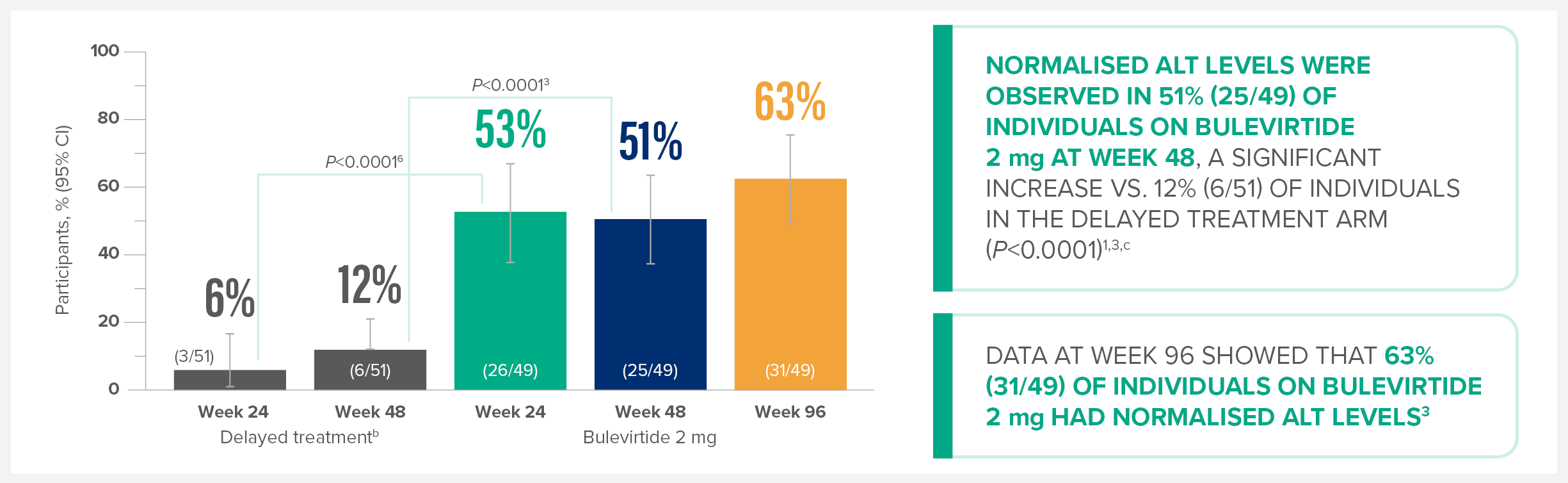
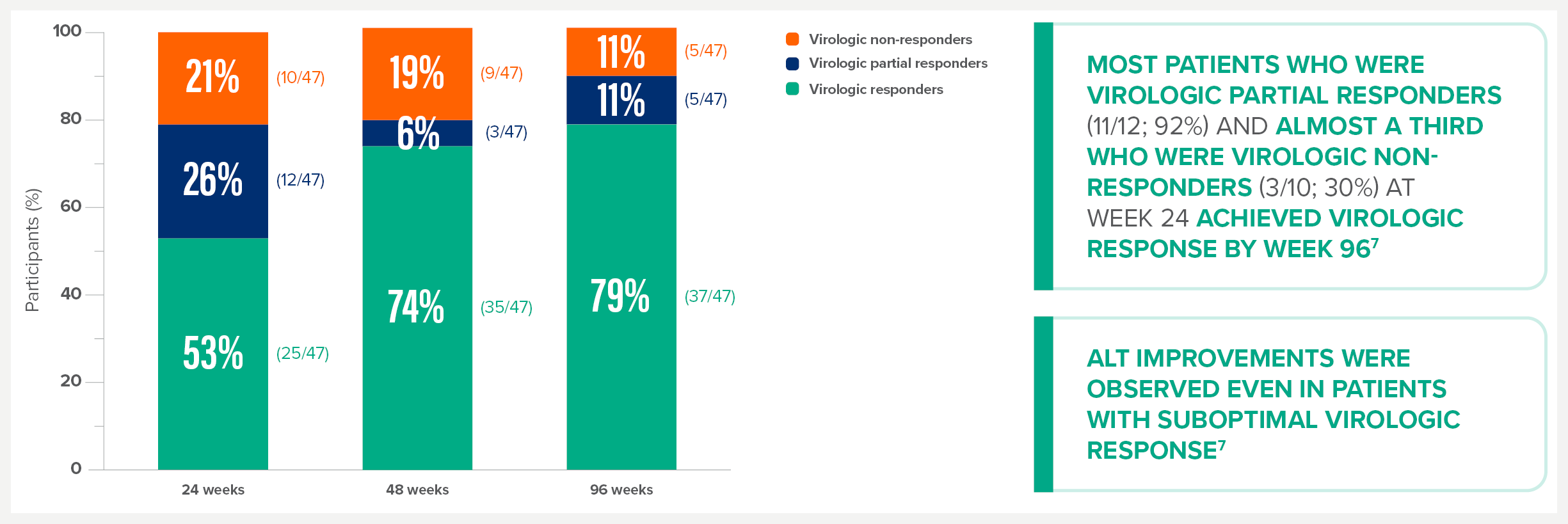
MYR301 is a completed, multicentre, open-label, randomised, Phase 3 study in adults with chronic HDV infection with or without cirrhosis (n=150).1
The primary objective of this study was to evaluate the safety and efficacy of HEPCLUDEX® for treatment of chronic HDV in comparison to delayed treatment. The study commenced in April 2019 and was completed in August 2024.2