CTP = Child-Turcotte-Pugh; DNA = deoxyribonucleic acid; HBV = hepatitis B virus; HCV = hepatitis C virus; HDV = hepatitis D virus; SmPC = Summary of Product Characteristics.
HEPCLUDEX® safety summary
Safety and tolerability profile1
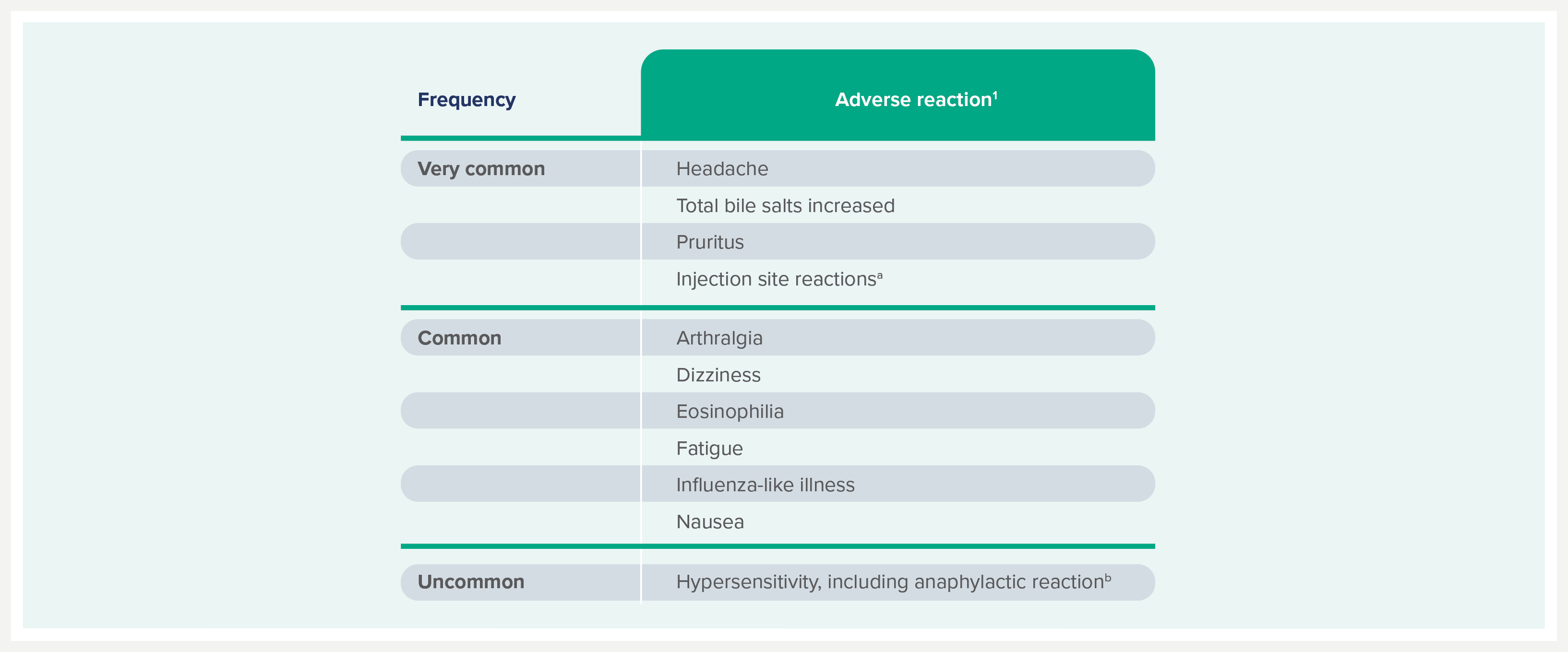
Pooled data from clinical studies and post-marketing experience.
The most frequently reported adverse reactions are headache, pruritus, injection site reactions, and increase in bile salts; increase in bile salts were usually asymptomatic and reversible after discontinuation of treatment.1
The most frequently reported serious adverse reaction is an exacerbation of hepatitis after discontinuation of HEPCLUDEX®, possibly related to virologic rebound after treatment discontinuation.1
Adverse reactions are listed by frequency. Frequencies are defined as follows: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100).
aInjection site events include injection site erythema, injection site reaction, injection site pain, injection site induration, injection site rash, injection site haematoma, injection site pruritus and injection site dermatitis.1
bAdverse reaction identified through post-marketing surveillance.1
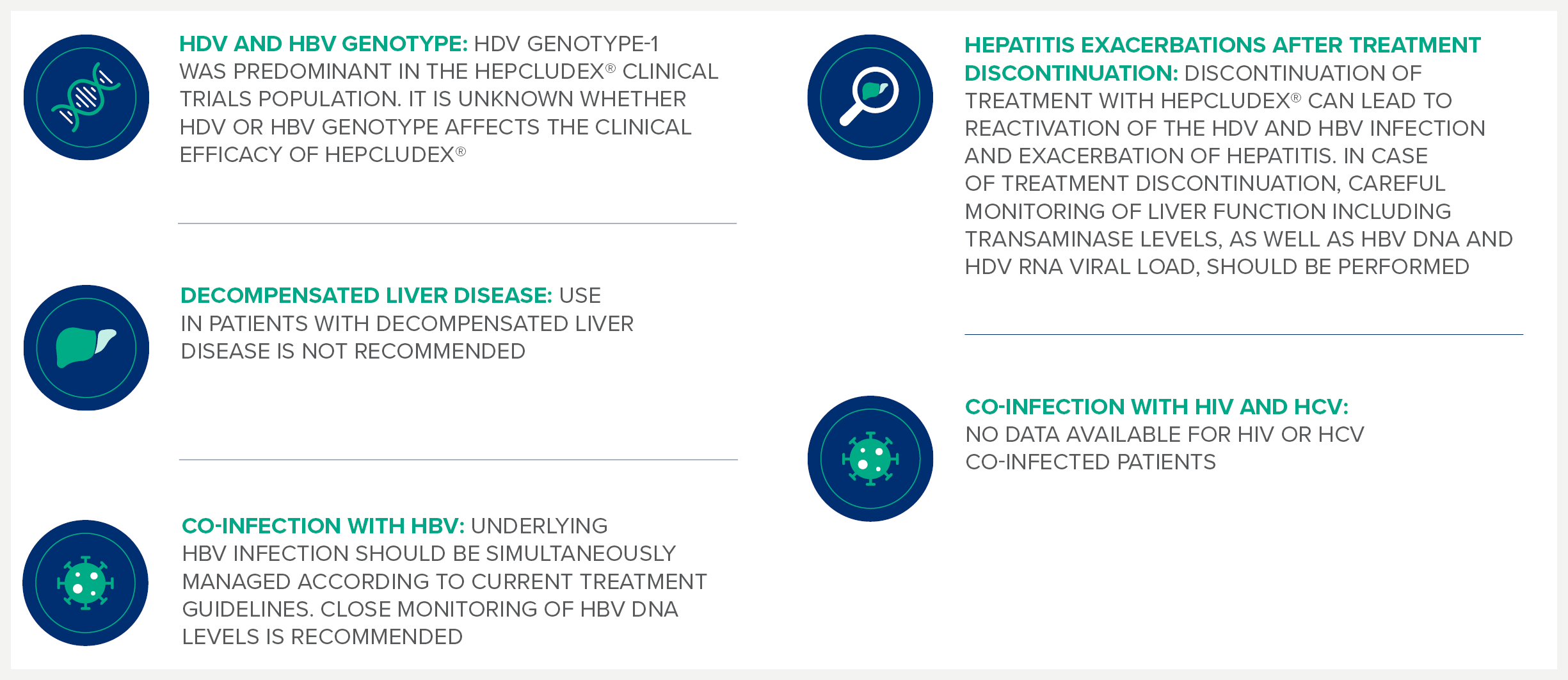
Special warnings and precautions1
You may also like to visit
Abbreviations:
UKI-HPX-0038
Date of preparation May 2025

